Gibbs energy and spontaneity :
`=>` We have seen that for a system, it is the total entropy change, `color{purple}(DeltaS_text(total))` which decides the spontaneity of the process. But most of the chemical reactions fall into the category of either closed systems or open systems.
● Therefore, for most of the chemical reactions there are changes in both enthalpy and entropy.
● It is clear that neither decrease in enthalpy nor increase in entropy alone can determine the direction of spontaneous change for these systems.
● For this purpose, we define a new thermodynamic function the Gibbs energy or Gibbs function, `color{purple}(G)`, as
`color{purple}(G = H - TS)` ..............(6.20)
● Gibbs function, `color{purple}(G)` is an extensive property and a state function.
● The change in Gibbs energy for the system `color{purple}(DeltaG_text(sys))` can be written as
`color{purple}(DeltaG_text(sys) = DeltaH_text(sys) - T DeltaS_text(sys) - S_text(sys) Delta T)`
● At constant temperature, `color{purple}(DeltaT = 0)`
`therefore color{purple}(DeltaG_text(sys) = DeltaH_text(sys) - T DeltaS_text(sys))`
● Usually the subscript ‘system’ is dropped and we simply write this equation as `color{purple}(DeltaG = DeltaH - T Delta S)` .........(6.21)
● Thus, Gibbs energy change = enthalpy change – temperature × entropy change, and is referred to as the Gibbs equation, one of the most important equations in chemistry.
● Here, we have considered both terms together for spontaneity : energy (in terms of `color{purple}(DeltaH)`) and entropy (`color{purple}(DeltaS)`, a measure of disorder) as indicated earlier.
● Dimensionally if we analyse, we find that` color{purple}(DeltaG)` has units of energy because, both `color{purple}(DeltaH)` and the `color{purple}(TDeltaS)` are energy terms, since `color{purple}(TDeltaS = (K) (J//K) = J)`.
`=>` Now let us consider how `color{purple}(DeltaG)` is related to reaction spontaneity. We know
`color{purple}(DeltaS_text(total) = DeltaS_text(sys)+DeltaS_text(surr))`
● If the system is in thermal equilibrium with the surrounding, then the temperature of the surrounding is same as that of the system.
● Also, increase in enthalpy of the surrounding is equal to decrease in the enthalpy of the system.
● Therefore, entropy change of surroundings,
`color{purple}(DeltaS_text(surr) = (DeltaH_text(surr))/T = - (DeltaH_text(sys))/T)`
`color{purple}(DeltaS_text(total) = DeltaS_text(sys) + ( -DeltaH_text(sys))/T)`
Rearranging the above equation :
`color{purple}(TDeltaS_text(total) = T DeltaS_text(sys) - DeltaH_text(sys))`
● For spontaneous process, `color{purple}(DeltaS_text(total) > 0 )`, so `color{purple}(T Delta S_text(sys) - DeltaH_text(sys) > 0)`
`=> - color{purple}(( DeltaH_text(sys) - T Delta S_text(sys) ) > 0)`
● Using equation 6.21, the above equation can be written as
`color{purple}(- DeltaG > 0)`
`color{purple}(DeltaG = DeltaH - T Delta S < 0)` ...........(6.22)
`=>` `color{purple}(DeltaH_text(sys))` is the enthalpy change of a reaction, `color{purple}(TDeltaS_text(sys))` is the energy which is not available to do useful work.
`=>` So `color{purple}(DeltaG)` is the net energy available to do useful work and is thus a measure of the ‘free energy’. For this reason, it is also known as the free energy of the reaction.
`=>` `color{purple}(DeltaG)` gives a criteria for spontaneity at constant pressure and temperature.
(i) If `color{purple}(DeltaG)` is negative `(< 0)`, the process is spontaneous.
(ii) If `color{purple}(DeltaG)` is positive `(> 0)`, the process is non spontaneous.
`color{red}("Note ")` If a reaction has a positive enthalpy change and positive entropy change, it can be spontaneous when `color{purple}(TDeltaS)` is large enough to outweigh `color{purple}(DeltaH)`. This can happen in two ways :
(a) The positive entropy change of the system can be ‘small’ in which case `color{purple}(T)` must be large.
(b) The positive entropy change of the system can be ’large’, in which case `color{purple}(T)` may be small.
● The former is one of the reasons why reactions are often carried out at high temperature.
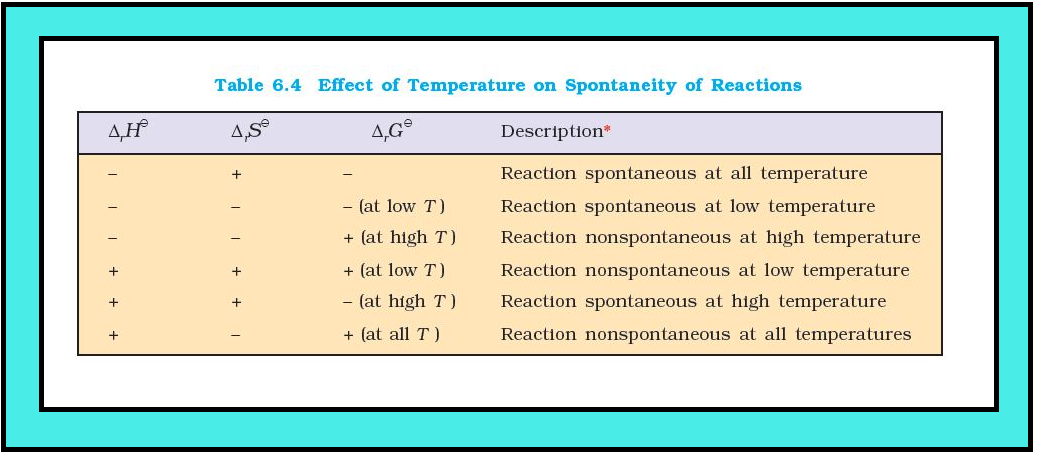
● Table 6.4 summarises the effect of temperature on spontaneity of reactions.
● Therefore, for most of the chemical reactions there are changes in both enthalpy and entropy.
● It is clear that neither decrease in enthalpy nor increase in entropy alone can determine the direction of spontaneous change for these systems.
● For this purpose, we define a new thermodynamic function the Gibbs energy or Gibbs function, `color{purple}(G)`, as
`color{purple}(G = H - TS)` ..............(6.20)
● Gibbs function, `color{purple}(G)` is an extensive property and a state function.
● The change in Gibbs energy for the system `color{purple}(DeltaG_text(sys))` can be written as
`color{purple}(DeltaG_text(sys) = DeltaH_text(sys) - T DeltaS_text(sys) - S_text(sys) Delta T)`
● At constant temperature, `color{purple}(DeltaT = 0)`
`therefore color{purple}(DeltaG_text(sys) = DeltaH_text(sys) - T DeltaS_text(sys))`
● Usually the subscript ‘system’ is dropped and we simply write this equation as `color{purple}(DeltaG = DeltaH - T Delta S)` .........(6.21)
● Thus, Gibbs energy change = enthalpy change – temperature × entropy change, and is referred to as the Gibbs equation, one of the most important equations in chemistry.
● Here, we have considered both terms together for spontaneity : energy (in terms of `color{purple}(DeltaH)`) and entropy (`color{purple}(DeltaS)`, a measure of disorder) as indicated earlier.
● Dimensionally if we analyse, we find that` color{purple}(DeltaG)` has units of energy because, both `color{purple}(DeltaH)` and the `color{purple}(TDeltaS)` are energy terms, since `color{purple}(TDeltaS = (K) (J//K) = J)`.
`=>` Now let us consider how `color{purple}(DeltaG)` is related to reaction spontaneity. We know
`color{purple}(DeltaS_text(total) = DeltaS_text(sys)+DeltaS_text(surr))`
● If the system is in thermal equilibrium with the surrounding, then the temperature of the surrounding is same as that of the system.
● Also, increase in enthalpy of the surrounding is equal to decrease in the enthalpy of the system.
● Therefore, entropy change of surroundings,
`color{purple}(DeltaS_text(surr) = (DeltaH_text(surr))/T = - (DeltaH_text(sys))/T)`
`color{purple}(DeltaS_text(total) = DeltaS_text(sys) + ( -DeltaH_text(sys))/T)`
Rearranging the above equation :
`color{purple}(TDeltaS_text(total) = T DeltaS_text(sys) - DeltaH_text(sys))`
● For spontaneous process, `color{purple}(DeltaS_text(total) > 0 )`, so `color{purple}(T Delta S_text(sys) - DeltaH_text(sys) > 0)`
`=> - color{purple}(( DeltaH_text(sys) - T Delta S_text(sys) ) > 0)`
● Using equation 6.21, the above equation can be written as
`color{purple}(- DeltaG > 0)`
`color{purple}(DeltaG = DeltaH - T Delta S < 0)` ...........(6.22)
`=>` `color{purple}(DeltaH_text(sys))` is the enthalpy change of a reaction, `color{purple}(TDeltaS_text(sys))` is the energy which is not available to do useful work.
`=>` So `color{purple}(DeltaG)` is the net energy available to do useful work and is thus a measure of the ‘free energy’. For this reason, it is also known as the free energy of the reaction.
`=>` `color{purple}(DeltaG)` gives a criteria for spontaneity at constant pressure and temperature.
(i) If `color{purple}(DeltaG)` is negative `(< 0)`, the process is spontaneous.
(ii) If `color{purple}(DeltaG)` is positive `(> 0)`, the process is non spontaneous.
`color{red}("Note ")` If a reaction has a positive enthalpy change and positive entropy change, it can be spontaneous when `color{purple}(TDeltaS)` is large enough to outweigh `color{purple}(DeltaH)`. This can happen in two ways :
(a) The positive entropy change of the system can be ‘small’ in which case `color{purple}(T)` must be large.
(b) The positive entropy change of the system can be ’large’, in which case `color{purple}(T)` may be small.
● The former is one of the reasons why reactions are often carried out at high temperature.
● Table 6.4 summarises the effect of temperature on spontaneity of reactions.